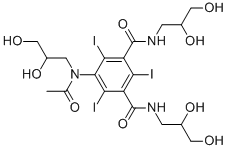
Iohexol
100 INR
Product Details:
- Melting Point 174-180 C
- Purity 98%
- Density 2.20.1 g/cm
- Usage This medication is used before X-ray imaging tests (such as CT scans). Iohexol contains iodine and belongs to a class of drugs known as contrast media or dyes. It works by adding contrast to body parts and fluids in these imaging tests
- Molecular Weight 821.138 Grams (g)
- Molecular Formula C19H26I3N3O
- Click to View more
X
Iohexol Price And Quantity
- 1 Kilograms
- 100 INR
Iohexol Product Specifications
- 174-180 C
- 98%
- 2.20.1 g/cm
- C19H26I3N3O
- 821.138 Grams (g)
- This medication is used before X-ray imaging tests (such as CT scans). Iohexol contains iodine and belongs to a class of drugs known as contrast media or dyes. It works by adding contrast to body parts and fluids in these imaging tests
Iohexol Trade Information
- Cash Advance (CA) Cash in Advance (CID)
- 500 Kilograms KG Per Month
- 2-8 Week
- Yes
- Sample costs shipping and taxes has to be paid by the buyer
- Carton and Poly Bag.
- Western Europe Australia Middle East Central America Africa South America Asia Eastern Europe North America
- All India
Product Description
Iohexol
analytical standard
Synonym: 5-
CAS Number 66108-95-0
Empirical Formula (Hill Notation) C19H26I3N3O9
Molecular Weight 821.14
Properties
| Related Categories | Additional Drugs, Additional Standards, Analytical Standards, Analytical/Chromatography, Chromatography, |
| grade | analytical standard |
| InChI Key | NTHXOOBQLCIOLC-UHFFFAOYSA |
| assay | 95.0% (HPLC) |
| form | neat |
| shelf life | limited shelf life, expiry date on the label |
| application(s) | HPLC: suitable |
| gas chromatography (GC): suitable | |
| impurities | 6.0% water |
| format | neat |
European Pharmacopoeia (EP) Reference Standard
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography, EP Standards, EP Standards I - K, Pharmacopeia & Metrological Institutes Standards |
| InChI Key | NTHXOOBQLCIOLC-UHFFFAOYSA |
| form | neat |
| format | neat |
United States Pharmacopeia (USP) Reference Standard
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography,Pharmacopeia & Metrological Institutes Standards, USP Standards, USP Standards I - K |
Enter Buying Requirement Details




![Dibenz[a,h]anthracene](https://cpimg.tistatic.com/03526048/b/4/Dibenz-a-h-anthracene.png?tr=w300)


 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free