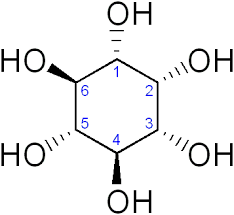
Inositol
Product Details:
- Purity 99%
- Melting Point 225 to 227 °C (437 to 441 °F; 498 to 500 K)
- Molecular Formula C6H12O6
- Physical Form Powder
- Usage Inositol is used for diabetic nerve pain, panic disorder, high cholesterol, insomnia, cancer, depression, schizophrenia, Alzheimer's disease, attention deficit-hyperactivity disorder (ADHD), autism, promoting hair growth, a skin disorder called psoriasis, and treating side effects of medical treatment with lithium.
- Molecular Weight 180.16 Grams (g)
- Click to View more
Inositol Price And Quantity
- 1 Kilograms
- 100 INR
Inositol Product Specifications
- Powder
- 99%
- Inositol is used for diabetic nerve pain, panic disorder, high cholesterol, insomnia, cancer, depression, schizophrenia, Alzheimer's disease, attention deficit-hyperactivity disorder (ADHD), autism, promoting hair growth, a skin disorder called psoriasis, and treating side effects of medical treatment with lithium.
- 225 to 227 °C (437 to 441 °F; 498 to 500 K)
- 180.16 Grams (g)
- C6H12O6
Inositol Trade Information
- Cash in Advance (CID) Cash Advance (CA)
- 500 Kilograms KG Per Month
- 2-8 Week
- Yes
- Sample costs shipping and taxes has to be paid by the buyer
- Carton and Poly Bag.
- Western Europe Eastern Europe Africa Middle East South America Asia Central America North America Australia
- All India
Product Description
Inositol
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym: 1,2,3,4,5,6-
CAS Number 87-89-8
Empirical Formula (Hill Notation) C6H12O6
Molecular Weight 180.16
Properties
| Related Categories | Additional Standards, Analytical Standards,Analytical/Chromatography, Chromatography,Pharmaceutical Secondary Standards, |
| pharmaceutical secondary standard | |
| vapor density | 6.2 (vs air) |
| InChI Key | CDAISMWEOUEBRE-GPIVLXJGSA |
| form | neat |
| mp | 222-227 C(lit.) |
| format | neat |
Description
Analysis Note
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
Biochem/physiol Actions
A component of membrane phospholipids, glycosylphosphatidylino
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
United States Pharmacopeia (USP) Reference Standard
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography,Pharmacopeia & Metrological Institutes Standards, USP Standards, USP Standards I - K |
| vapor density | 6.2 (vs air) |
| InChI Key | CDAISMWEOUEBRE-GPIVLXJGSA |
| mp | 222-227 °C(lit.) |






 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free