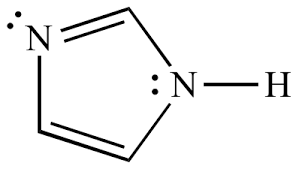
Imidazole
Product Details:
- Usage Histidine is present in many proteins and enzymes and plays a vital part in the structure and binding functions of hemoglobin. Imidazole-based histidine compounds play a very important role in intracellular buffering. Histidine can be decarboxylated to histamine, which is also a common biological compound.
- Melting Point 89 to 91 C (192 to 196 F; 362 to 364 K)
- Molecular Weight 68.077 Grams (g)
- Molecular Formula C3H4N2
- Appearance white or pale yellow solid
- Click to View more
Imidazole Price And Quantity
- 100 INR
- 1 Kilograms
Imidazole Product Specifications
- 68.077 Grams (g)
- 89 to 91 C (192 to 196 F; 362 to 364 K)
- Histidine is present in many proteins and enzymes and plays a vital part in the structure and binding functions of hemoglobin. Imidazole-based histidine compounds play a very important role in intracellular buffering. Histidine can be decarboxylated to histamine, which is also a common biological compound.
- C3H4N2
- white or pale yellow solid
Imidazole Trade Information
- Cash in Advance (CID) Cash Advance (CA)
- 500 Kilograms KG Per Month
- 2-8 Week
- Yes
- Sample costs shipping and taxes has to be paid by the buyer
- Carton and Poly Bag.
- Western Europe Australia Middle East Central America Africa South America Asia Eastern Europe North America
- All India
Product Description
Imidazole
95.0% (HPLC), pharmaceutical impurity standard
Synonym: 1,3-
CAS Number 288-32-4
Empirical Formula (Hill Notation) C3H4N2
Molecular Weight 68.08
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography,Ondansetron hydrochloride Impurities, Pharmaceutical Impurities, Pharmacopeia & Metrological Institutes Standards |
| grade | analytical standard |
| vapor pressure | <1 mmHg ( 20 C) |
| InChI Key | RAXXELZNTBOGNW-UHFFFAOYSA |
| assay | 95.0% (HPLC) |
| shelf life | limited shelf life, expiry date on the label |
| impurities | 10.0% related substances |
| 5.0% solvents | |
| 5.0% water | |
| pH | 9-11 at 100 g/L (23 C) |
| pKa (25 C) | 6.95 |
| bp | 256 C(lit.) |
| mp | 88-91 C(lit.) |
| storage temp. | 2-8C |
United States Pharmacopeia (USP) Reference Standard
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography,Pharmacopeia & Metrological Institutes Standards, USP Standards, USP Standards I - K |
| grade | USP reference standard |
| vapor pressure | <1 mmHg ( 20 C) |
| InChI Key | RAXXELZNTBOGNW-UHFFFAOYSA |
| form | neat |
| pKa (25 C) | 6.95 |
| bp | 256 C(lit.) |
| mp | 88-91 C(lit.) |
| format | neat |
Description
Application
Excellent for buffers in the range of pH 6.2-7.8
Pharmaceutical Secondary Standard;
Properties
| Related Categories | Additional Standards, Analytical Standards,Analytical/Chromatography, Chromatography, Clotrimazole Impurities, |
| grade | certified reference material |
| pharmaceutical secondary standard | |
| vapor pressure | <1 mmHg ( 20 C) |
| InChI Key | RAXXELZNTBOGNW-UHFFFAOYSA |
| form | neat |
| pKa (25 C) | 6.95 |
| bp | 256 C(lit.) |
| mp | 88-91 C(lit.) |
| format | neat |
Description
Analysis Note
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
Application
Excellent for buffers in the range of pH 6.2-7.8
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.








 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free