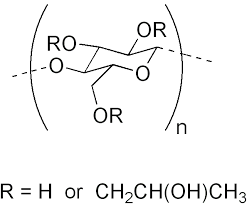
Hydroxypropyl cellulose
100 INR
Product Details:
- Molecular Formula C56H108O30
- Melting Point 100-150° C
- Usage Lacrisert, manufactured by Aton Pharma, is a formulation of HPC used for artificial tears. It is used to treat medical conditions characterized by insufficient tear production such as keratoconjunctivitis sicca), recurrent corneal erosions, decreased corneal sensitivity, exposure and neuroparalytic keratitis.
- Molecular Weight 806.9 Grams (g)
- Appearance white to slightly yellow-colored, odorless and tasteless powder.
- Click to View more
X
Hydroxypropyl cellulose Price And Quantity
- 1 Kilograms
- 100 INR
Hydroxypropyl cellulose Product Specifications
- white to slightly yellow-colored, odorless and tasteless powder.
- Lacrisert, manufactured by Aton Pharma, is a formulation of HPC used for artificial tears. It is used to treat medical conditions characterized by insufficient tear production such as keratoconjunctivitis sicca), recurrent corneal erosions, decreased corneal sensitivity, exposure and neuroparalytic keratitis.
- 100-150° C
- C56H108O30
- 806.9 Grams (g)
Hydroxypropyl cellulose Trade Information
- Cash in Advance (CID) Cash Advance (CA)
- 500 Kilograms KG Per Month
- 2-8 Week
- Yes
- Sample costs shipping and taxes has to be paid by the buyer
- Carton and Poly Bag.
- Western Europe Eastern Europe Central America Africa Middle East South America Asia North America Australia
- All India
Product Description
Hydroxypropyl cellulose
United States Pharmacopeia (USP) Reference Standard
CAS Number 9004-64-2
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography,Pharmacopeia & Metrological Institutes Standards, USP Standards, USP Standards G - H |
| form | neat |
| autoignition temp. | 752 F |
| solubility | DMF: soluble |
| DMSO: soluble | |
| H2O: insoluble (hot) | |
| H2O: soluble (cold) | |
| alcohol: soluble | |
| chloroform: soluble | |
| dioxane: soluble | |
| isopropanol: soluble | |
| methanol: soluble | |
| propylene glycol: soluble | |
| density | 0.5 g/mL at 25 C(lit.) |
| format | neat |
European Pharmacopoeia (EP) Reference Standard
Properties
| Related Categories | Analytical Standards, Analytical/Chromatography, EP Standards, EP Standards G - H, Pharmacopeia & Metrological Institutes Standards |
| autoignition temp. | 752 °F |
| density | 0.5 g/mL at 25 °C(lit.) |
Pharmaceutical Secondary Standard; Certified Reference Material
Properties
| Related Categories | Additional Standards, Analytical Standards,Analytical/Chromatography, Chromatography,Pharmaceutical Secondary Standards, |
| pharmaceutical secondary standard | |
| autoignition temp. | 752 °F |
| packaging | pkg of 1 g |
| density | 0.5 g/mL at 25 °C(lit.) |
Description
Analysis Note
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Enter Buying Requirement Details







 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free