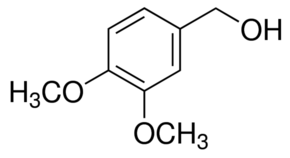
3,4-Dimethoxybenzyl alcohol (Verapamil Related Compound F - USP)
100 INR
Product Details:
- Purity 99%
- Molecular Formula C9H12O3
- Usage 3,4-Dimethoxybenzyl alcohol is widely used in the synthesis of various cyclotriveratrylenes (CTVs), which are cyclic molecular hosts with a cavity to accommodate guest molecules. It can also be used as a precursor in the total synthesis of salvianolic acid N.
- Melting Point 46-50°C
- Molecular Weight 168.192 Grams (g)
- Click to View more
X
3,4-Dimethoxybenzyl alcohol (Verapamil Related Compound F - USP) Price And Quantity
- 100 INR
- 1 Kilograms
3,4-Dimethoxybenzyl alcohol (Verapamil Related Compound F - USP) Product Specifications
- 99%
- C9H12O3
- 168.192 Grams (g)
- 3,4-Dimethoxybenzyl alcohol is widely used in the synthesis of various cyclotriveratrylenes (CTVs), which are cyclic molecular hosts with a cavity to accommodate guest molecules. It can also be used as a precursor in the total synthesis of salvianolic acid N.
- 46-50°C
3,4-Dimethoxybenzyl alcohol (Verapamil Related Compound F - USP) Trade Information
- Cash in Advance (CID) Cash Advance (CA)
- 500 Kilograms Per Month
- 3-4 Week
- Yes
- Sample costs shipping and taxes has to be paid by the buyer
- Western Europe Australia Central America Middle East South America Asia Eastern Europe North America Africa
- All India
Product Description
3,4-Dimethoxybenzyl alcohol (Verapamil Related Compound F - USP)
pharmaceutical secondary standard; traceable to USP
Synonym: 3,4-Dimethoxybenzyl alcohol, Verapamil related compound F, Veratryl alcohol
-
CAS Number 93-03-8
-
Linear Formula (CH3O)2C6H3CH2OH
-
Molecular Weight 168.19
| grade | certified reference material |
| pharmaceutical secondary standard | |
| form | neat |
| refractive index | n20/D 1.552(lit.) |
| bp | 296-297 °C/732 mmHg(lit.) |
| density | 1.157 g/mL at 25 °C(lit.) |
| format | neat |
| pharmacopeia traceability | traceable to USP 1711440 |
Analysis Note
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
General description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Enter Buying Requirement Details





 Send Inquiry
Send Inquiry Send SMS
Send SMS Call Me Free
Call Me Free